Nicotine Drug Test for Smoking & Vaping (Cotinine)
From £2.49
• Fast results in 5 minutes
• Detects tobacco use up to 4 days after smoking
• Laboratory-grade accuracy (200 ng/ml cutoff)
• Clear positive/negative results
• Complete kit with all testing materials
• Suitable for home or professional use
This test kit lets you check if someone has used tobacco. It looks for cotinine in urine – this is what nicotine turns into in your body. The test can spot cotinine levels of 200 ng/ml or higher. Cotinine usually stays in your body for 2-4 days after smoking.
Why We Use Cotinine Tests
Healthcare providers, employers, and insurance companies rely on cotinine tests because they’re accurate and hard to fool. Unlike questionnaires or interviews, these tests give clear yes-or-no answers about tobacco use. While some might try to beat the test, lab technicians know all the common tricks. Most importantly, these tests help doctors give better care – especially before surgery or when monitoring smoking-related health issues. For researchers, cotinine testing provides reliable data about smoking patterns in different groups.
How Long Does Cotinine Stay in Your System?
We test for cotinine instead of nicotine because it stays in your body longer. While nicotine leaves within hours, cotinine remains for days. Heavy smokers might test positive for up to a week. Light smokers may clear the test in 2 days. Your age, diet, and medicines can affect how quickly cotinine leaves your body.
For best results, test your first morning urine. Remember that being around smokers can sometimes cause a positive result.
Health Risks of Smoking
Smoking is the biggest cause of early death that we can prevent. No tobacco product is safe. Smoking can cause:
- Heart disease
- Lung cancer
- Other serious health problems
At least 1 in 5 heart disease deaths link to smoking.
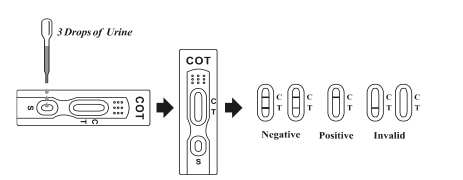
How to Use the Test
- Let the test kit reach room temperature
- Put 3 drops of urine in the test well
- Wait 5 minutes to read the result
- Don’t read results after 10 minutes
What Your Results Mean
- Two lines = Negative (below 200 ng/ml)
- One line in ‘C’ area = Positive (above 200 ng/ml)
- No line in ‘C’ area = Test didn’t work, try again
Important Notes
- Store the test at room temperature
- Don’t use after expiry date
- Keep the test sealed until use
- Treat urine samples with care
- Throw away used tests properly
Remember: This is just a first check. For certain results, you’ll need lab testing.







Reviews
There are no reviews yet